One of the more recent, significant patent litigation cases within biotechnology is the Association for Molecular Pathology v. Myriad Genetics decided by the Supreme Court in 2013.
In this case, the Association for Molecular Pathology challenged Myriad Genetics for its patents relating to BRCA1 and BRCA2 genes and particular mutations in these genes that are strongly associated with the development of breast cancer. As noted by the majority opinion in this case,
"the average American woman has a 12- to 12-percent risk of developing breast cancer, but for women with certain genetic mutations, the risk can range between 50 and 80 percent for breast cancer and between 20 and 50 percent for ovarian cancer (2)."
The specific patents in question regard mutation diagnostic methods that allowed Myriad to exclusively control diagnostic testing and scientific research on the BRCA genes. Myriad received these patents based on its research
The plaintiff, the Association for Molecular Pathology, argued that the patents on the BRCA1/2 genes are patents on products of nature. Since Section 101 of Title 35 of the United States Code defines patentable subject matter as
"any new and useful process, machine, manufacture, or compositions of matter, or any new and useful improvement thereof"
if the genes claimed are merely products of nature, they are not patent eligible. Therefore, the plaintiffs claimed that Myriad Genetics' patents are invalid based on Section 101. Myriad's defense is based on the argument that its patents are valid because once a gene is isolated, it is distinguishable from other genes.
Additionally, the plaintiffs noted that Myriad's exclusivity in regards to BRCA research is an impediment to further scientific research.
This case originated at the district court, which ruled in favor of the Association for Molecular Pathology, invalidating Myriad Genetics BRCA patents. Myriad appealed this case to the U.S. Court of Appeals for the Federal Circuit. The appeal court reversed the district court decision and held Myriad's patents valid on the grounds that isolated genes are chemically and structurally distinct from genes in the body. The Association for Molecular Pathology appealed this decision to the U.S. Supreme Court where in a unanimous 9-0 decision, SCOTUS decided that naturally occurring gene sequences are not patent eligible, however gene sequences that are not occurring in nature are indeed patent eligible. This decision reverses in part and affirms in part the decision of the appeals court.
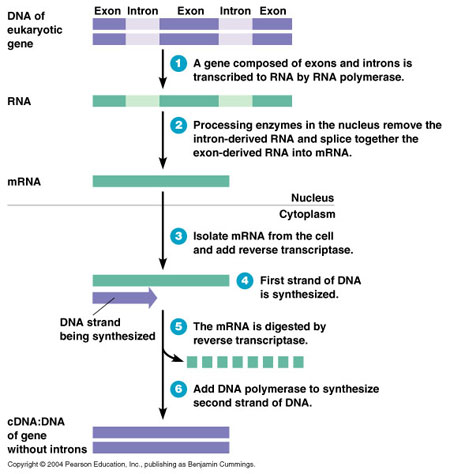
To understand the two-fold implications of this Supreme Court decision, some molecular biology background is required. Genes are sequences of DNA. This case sets the precedent that naturally occurring sequences of DNA cannot be patented because they are products of nature, and merely isolation does not, on its own, make these sequences of DNA patent eligible. However, there are sequences of DNA that are not occurring naturally in nature. The prime example of these sequences include cDNA. cDNA is DNA that is made in laboratory settings, excluding introns - noncoding regions of DNA.
Therefore, this decision sets precedent for gene patents in that naturally occurring sequences cannot be patented, however cDNA can be patented.
Sources:
(1) https://www.ocf.berkeley.edu/~edy/genomics/cdna.jpg
(2) https://www.oyez.org/cases/2012/12-398
(3) http://i2.cdn.turner.com/cnnnext/dam/assets/130523190137-sulik-myriad-genetics-protest-horizontal-large-gallery.jpg